Amino Acids That Can Form Hydrogen Bonds - A) arginine and glutamic acid.
Amino Acids That Can Form Hydrogen Bonds - Web polar amino acids (form hydrogen bonds as proton donors or acceptors): Web which amino acids can form hydrogen bonds? Web there are 20 types of amino acids commonly found in proteins. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Each turn of the helix spans 3.6 amino acids.
Amino acids share a basic structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group ( nh 2 ), a carboxyl group ( cooh ), and a hydrogen atom. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. There are two requirements for hydrogen bonding. Web the carbonyl group can function as a hydrogen bond acceptor, and the amino group (nh 2) can function as a hydrogen bond donor. Web which amino acids can form hydrogen bonds? Hydrophobic side chains interact with each other via weak van der waals. A hydrogen atom (check) bonded to an electronegative atom (typically $\ce{o, n, f}$ — check) and another electronegative atom that can receive (typically the same atoms — check) so a hydrogen bond is possible where an ionic interaction is not.
amino acids salt bridge vs hydrogen bond Chemistry Stack Exchange
Ion pairing is one of the most important noncovalent forces in chemistry, in. There are two requirements for hydrogen bonding. A few biologically important derivatives of the standard amino acids are shown in the figure below. Note that the side chains (represented as green spheres) point out from the helix. Such a bond is weaker.
Solved 18. The side chain of which amino acid can form
Tertiary structure is stabilized by a combination of forces, including. Web amino acids with polar groups that form hydrogen bonds to water are classified as. Web the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. The amino acids lysine, arginine, and histidine have side chains with charged basic groups..
Amino acids physical, chemical properties and peptide bond
Web which amino acids can form hydrogen bonds? The method could be used not only for synthesizing amides from carboxylic. Tertiary structure is stabilized by a combination of forces, including. Web a hydrogen bond is a very viable possibility, though. Web the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and.
(a) Hydrogen bonding patterns that describe the αand πconfigurations
Hydrogen bonds can exist between atoms in different molecules or in the same molecule. Web there are 20 types of amino acids commonly found in proteins. Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. Molecule which bears charged groups of opposite polarity. Example.
Two amino acids are joined together by
The amino acids lysine, arginine, and histidine have side chains with charged basic groups. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving.
PPT Introduction to Amino Acids of Medical Importance PowerPoint
Web the folding of a protein chain is, however, further constrained by many different sets of weak noncovalent bonds that form between one part of the chain and another. (figure 1) draw it as it would. Web the carbonyl group can function as a hydrogen bond acceptor, and the amino group (nh 2) can function.
Chapter 22 Presentation
What amino acid participated in disulfide bonds? Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. They do not.
organic chemistry Which atoms in a given amino acid are able to form
Hydrophobic side chains interact with each other via weak van der waals. A hydrogen atom (check) bonded to an electronegative atom (typically $\ce{o, n, f}$ — check) and another electronegative atom that can receive (typically the same atoms — check) so a hydrogen bond is possible where an ionic interaction is not. Ion pairing is.
PPT Inner Life of a Cell PowerPoint Presentation, free download ID
Ion pairing is one of the most important noncovalent forces in chemistry, in. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the.
PPT Proteins PowerPoint Presentation, free download ID1828850
The weak bonds are of three types: Tertiary structure is stabilized by a combination of forces, including. These form hydrogen bonds to a purine, pyrimidine, or phosphate group in dna. Hydrogen bonds, ionic bonds, and van der waals. (figure 1) draw it as it would. Please explain why that is the correct answer. They do.
Amino Acids That Can Form Hydrogen Bonds A) arginine and glutamic acid. They do not ionize in normal conditions, a prominent exception being the catalytic serine in serine proteases. Hydrogen bonding and ionic bonding (figure 1). C) aspartic acid and lysine. Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group.
What Amino Acid Participated In Disulfide Bonds?
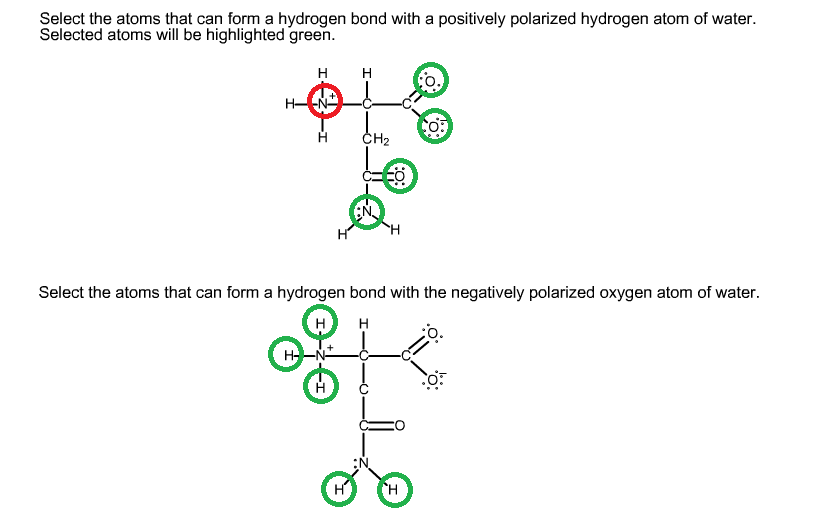
Web which pair of amino acids can form hydrogen bonds between their r groups? Web because the polar side chains of these amino acids can form hydrogen bonds with water, these amino acids are hydrophilic and tend to be located on the outside of proteins. For this problem, draw all hydrogen atoms explicitly. The weak bonds are of three types:
The Amino Acids Lysine, Arginine, And Histidine Have Side Chains With Charged Basic Groups.
There are two requirements for hydrogen bonding. Web the folding of a protein chain is, however, further constrained by many different sets of weak noncovalent bonds that form between one part of the chain and another. Web 1 day agoscience biochemistry proteins are made from chains of amino acids. Which amino acids are involved in turns and kinks?
A Hydrogen Atom (Check) Bonded To An Electronegative Atom (Typically $\Ce{O, N, F}$ — Check) And Another Electronegative Atom That Can Receive (Typically The Same Atoms — Check) So A Hydrogen Bond Is Possible Where An Ionic Interaction Is Not.
These form hydrogen bonds to a purine, pyrimidine, or phosphate group in dna. The forces in secondary structure primarily involve hydrogen bonds. Molecule which bears charged groups of opposite polarity. This is an example of severe perturbation, and is not.
Conditional Amino Acids Include Arginine, Cysteine, Glutamine, Glycine, Proline, And Tyrosine.
Note that the side chains (represented as green spheres) point out from the helix. The amino acids are joined together by structures called peptide bonds. Amino acids share a basic structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group ( nh 2 ), a carboxyl group ( cooh ), and a hydrogen atom. Web which amino acids can form hydrogen bonds.