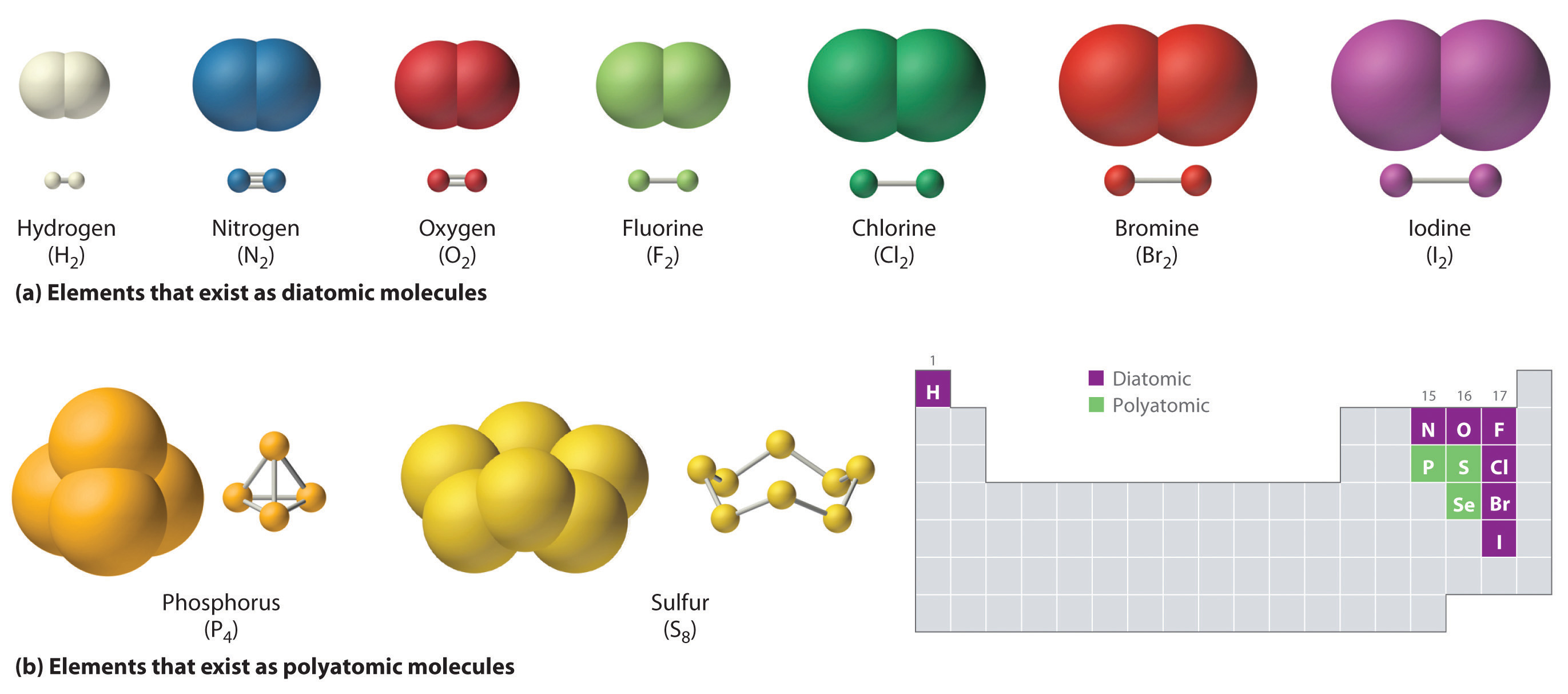
How Many Covalent Bonds Can Phosphorus Form - Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent.
How Many Covalent Bonds Can Phosphorus Form - Web phosphorus is a nonmetallic element having an atomic number of 15. Group 5a form 3 bonds; If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. It has 5 valence electrons, which means that it can form 3 covalent bonds.at most, the. Information starts using one straightforward picture off the standalone covalent bond.
Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web the halogens also form single covalent bonds to produce diatomic molecules. In each case, the sum. This is summarized in the table below. And group 7a form one bond. Web covalent bonds involve the sharing of electron pairs between atoms. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3.
Reading Covalent Bonds Biology I
Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. Web typically, the atoms of group 4a form 4 covalent bonds; Web the halogens.
Chemical Bonds · Anatomy and Physiology
Group 5a form 3 bonds; Web the halogens also form single covalent bonds to produce diatomic molecules. Web covalent bonds involve the sharing of electron pairs between atoms. Web typically, the atoms of group 4a form 4 covalent bonds; This is summarized in the table below. And group 7a form one bond. If the atoms.
covalent bond Definition, Properties, Examples, & Facts Britannica
And group 7a form one bond. Web how many bonds does phosphorus typically make? Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. Group 5a form 3 bonds; Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. Is determined by the distance at which the lowest potential.
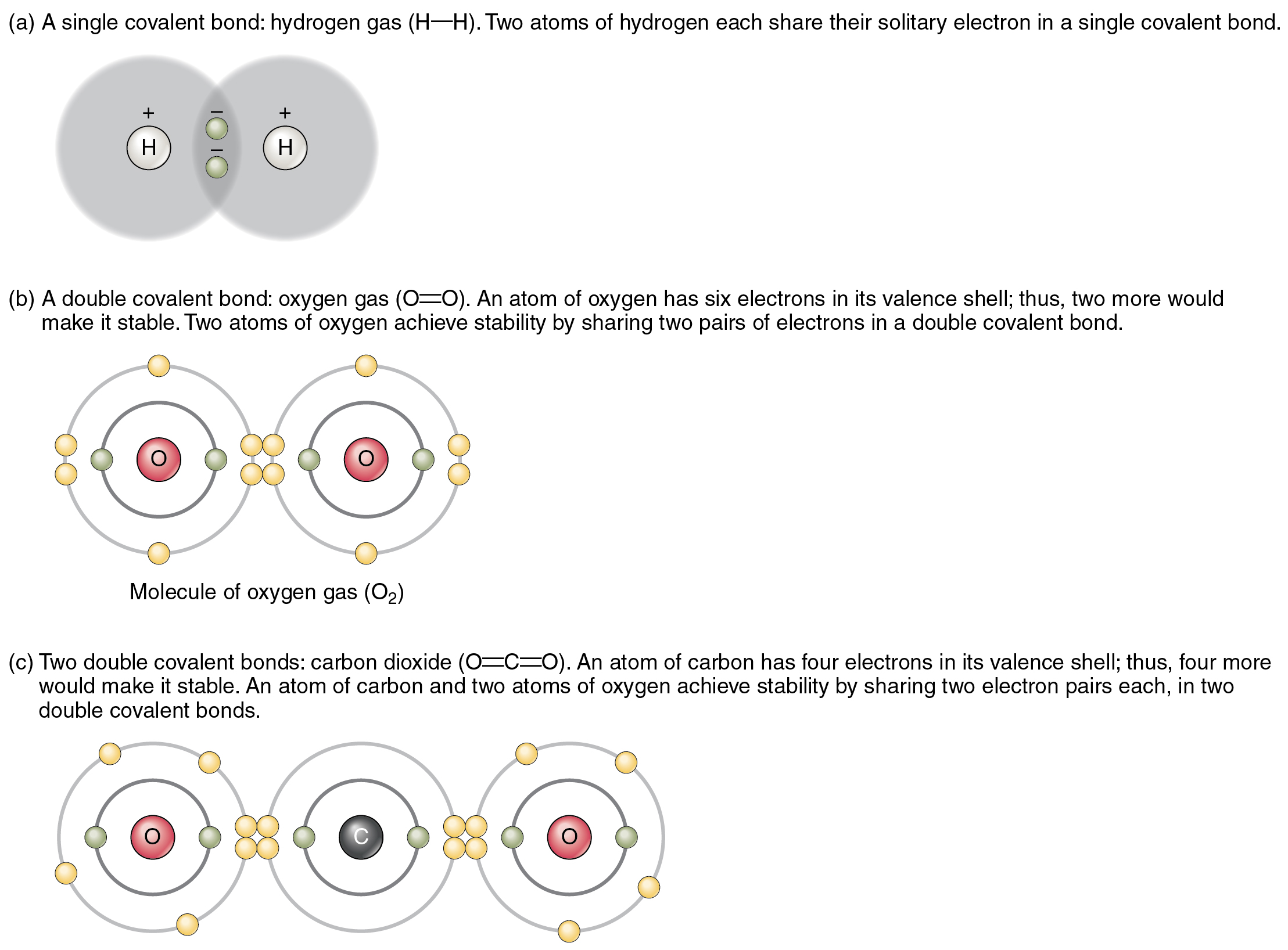
Covalent bonding in an oxygen molecule. Chemistry Activities, Gcse
Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. Web phosphorus is a nonmetallic element having an atomic number of 15. Information starts using one straightforward picture off the standalone covalent bond. In each case, the sum. Web molecular shapes & valence bond theory (0) worksheet. Web.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web how many bonds does phosphorus typically make? Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. This is summarized in the table below. It has 5 valence electrons, which means that it can form 3 covalent bonds.at most, the. Group 5a form.
Facts About Phosphorus Live Science
Web how many bonds does phosphorus typically make? Group 5a form 3 bonds; Similarly, the phosphorus atom in \(\ce{pcl_5}\) has 10 valence. Web typically, the atoms of group 4a form 4 covalent bonds; An atom of any halogen, such as fluorine, has seven valence electrons. Web covalent bonds involve the sharing of electron pairs between.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared. In each case, the sum. Valence shell electron pair repulsion theory (0) equatorial and axial positions (0) electron geometry (0). Similarly, the phosphorus atom in \(\ce{pcl_5}\).
41. What is the Lewis dot structure of phosphate ion? how many
Is determined by the distance at which the lowest potential energy is achieved. Valence shell electron pair repulsion theory (0) equatorial and axial positions (0) electron geometry (0). And group 7a form one bond. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. The electron configuration of the phosphorus atom can be represented.
The Expanded Octet Introduction to Chemistry
Group 5a form 3 bonds; Web (other elements, such as phosphorus [p] and cobalt [co], are able to form Web there are many different modifications of phosphorus in nature. Web typically, the atoms of group 4a form 4 covalent bonds; If the atoms that form a covalent bond are identical, as in h 2, cl.
Chemical Bonds · Anatomy and Physiology
Group 6a form 2 bonds; Web the halogens also form single covalent bonds to produce diatomic molecules. Web how many bonds does phosphorus typically make? Web molecular shapes & valence bond theory (0) worksheet. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. The potential energy of two separate hydrogen atoms (right) decreases.
How Many Covalent Bonds Can Phosphorus Form Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. The potential energy of two separate hydrogen atoms (right) decreases as. In each case, the sum. Web the halogens also form single covalent bonds to produce diatomic molecules. Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds.
Web There Are Many Different Modifications Of Phosphorus In Nature.
Web this page explains what covalent bonding is. It has 5 valence electrons, which means that it can form 3 covalent bonds.at most, the. Is determined by the distance at which the lowest potential energy is achieved. Web phosphorus is a nonmetallic element having an atomic number of 15.
Group 5A Form 3 Bonds;
Web the halogens also form single covalent bonds to produce diatomic molecules. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent. Web the number of valence electrons in phosphorus is generally 5 and it requires 3 more electrons to complete its octet. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared.
Group 6A Form 2 Bonds;
And group 7a form one bond. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. And group 7a form one bond. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
If The Atoms That Form A Covalent Bond Are Identical, As In H 2, Cl 2, And Other Diatomic Molecules, Then The Electrons In The Bond Must Be Shared.
Information starts using one straightforward picture off the standalone covalent bond. Group 6a form 2 bonds; Web how many bonds does phosphorus typically make? Group 5a form 3 bonds;