Identify The Keto Form Of Each Enol Tautomer - Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group.
Identify The Keto Form Of Each Enol Tautomer - Alkehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical. Web which of the following have an enol form?i. Web the individual keto and enol isomers are called tautomers. Identify the enol form of each keto tautomer. Note the difference between tautomers and resonance forms.
Web propanal is 1000 times more likely to be in its enol tautomer than acetone. Web the individual keto and enol isomers are called tautomers. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. It can spontaneously through equilibrium get to the actual enol form. Acids and bases both bring about the. Web in a solution, you won't see much of the enol form, but these can occur. You'll get a detailed solution from a subject matter expert.
Solved Identify the enol form of each keto tautomer.
You'll get a detailed solution from a subject matter expert. Web they are independent species in equilibrim with each other. Identify the enol form of each keto tautomer. 11.30 draw the enol form of each keto tautomer in parts (a) and (b), and the keto form of each enol tautomer in darts (c) and. Tautomers.
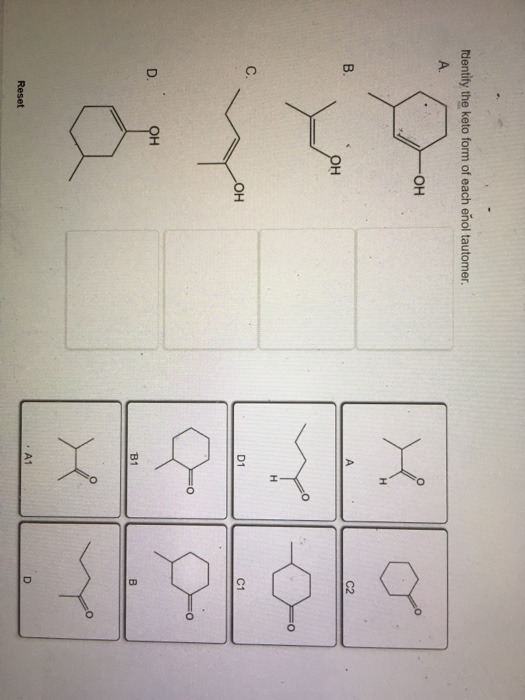
Solved dentify the keto form of each enol tautomer он C2 C.
Web the keto form and the enol form, and these are different molecules. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Web the individual keto and enol isomers are called tautomers. Web in a solution, you won't see much of the enol form, but these can.
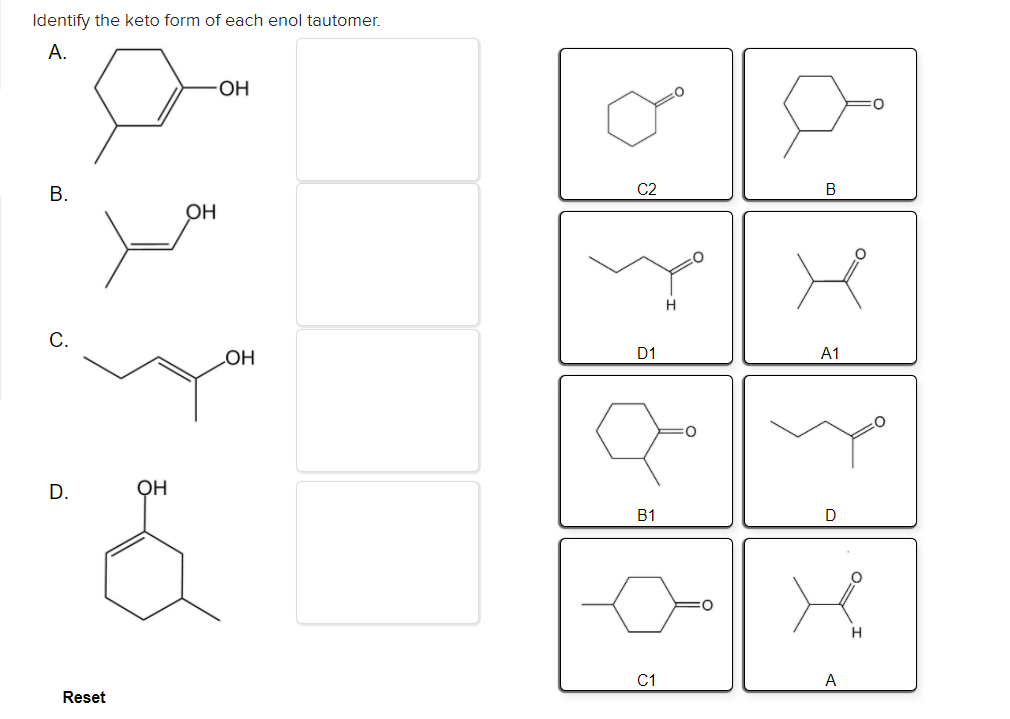
Solved Identify the keto form of each enol tautomer. А. ОН
Note the difference between tautomers and resonance forms. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Identify the enol form of each keto tautomer. Web the individual keto and enol isomers are called tautomers. To be tautomers, the two species must feature a central carbon which.
KetoEnol Tautomerism Key Points Master Organic Chemistry
Web propanal is 1000 times more likely to be in its enol tautomer than acetone. To be tautomers, the two species must feature a central carbon which in the enol form. Web in a solution, you won't see much of the enol form, but these can occur. 11.30 draw the enol form of each keto.
KetoEnol Tautomerism Key Points Master Organic Chemistry
Identify the enol form of each keto tautomer. Web which of the following have an enol form?i. Acids and bases both bring about the. This problem has been solved! Web they are independent species in equilibrim with each other. Web the individual keto and enol isomers are called tautomers. To be tautomers, the two species.
Solved 12. Which of the following is a ketoenol tautomeric
You'll get a detailed solution from a subject matter expert. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group. Web which of the following have an enol form?i. They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Note.
Keto Enol Tautomerism What Is It and Why Is It Important?
Web they are independent species in equilibrim with each other. To be tautomers, the two species must feature a central carbon which in the enol form. Web in a solution, you won't see much of the enol form, but these can occur. It can spontaneously through equilibrium get to the actual enol form. Web the.
KetoEnol Tautomerism Key Points Master Organic Chemistry
They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Acids and bases both bring about the. Note the difference between tautomers and resonance forms. Web the individual keto and enol isomers are called tautomers. Web the keto form and the enol form, and these are different molecules..
Solved Problem 11.11 Draw the keto tautomer of each enol. a.
Web in a solution, you won't see much of the enol form, but these can occur. Web propanal is 1000 times more likely to be in its enol tautomer than acetone. Web they are independent species in equilibrim with each other. It can spontaneously through equilibrium get to the actual enol form. Web the keto.
Keto Enol Tautomerization Reaction and Mechanism in Acid and Base
11.30 draw the enol form of each keto tautomer in parts (a) and (b), and the keto form of each enol tautomer in darts (c) and. Identify the enol form of each keto tautomer. Web propanal is 1000 times more likely to be in its enol tautomer than acetone. This problem has been solved! You'll.
Identify The Keto Form Of Each Enol Tautomer Identify the enol form of each keto tautomer. Web they are independent species in equilibrim with each other. Web in a solution, you won't see much of the enol form, but these can occur. 11.30 draw the enol form of each keto tautomer in parts (a) and (b), and the keto form of each enol tautomer in darts (c) and. To be tautomers, the two species must feature a central carbon which in the enol form.
Web Propanal Is 1000 Times More Likely To Be In Its Enol Tautomer Than Acetone.
They're isomers of each other so we call them tautomers and they're in equilibrium with each other. Acids and bases both bring about the. Note the difference between tautomers and resonance forms. You'll get a detailed solution from a subject matter expert.
It Can Spontaneously Through Equilibrium Get To The Actual Enol Form.
Web the individual keto and enol isomers are called tautomers. Web which of the following have an enol form?i. Identify the enol form of each keto tautomer. Web they are independent species in equilibrim with each other.
Web In A Solution, You Won't See Much Of The Enol Form, But These Can Occur.
Identify the enol form of each keto tautomer. 11.30 draw the enol form of each keto tautomer in parts (a) and (b), and the keto form of each enol tautomer in darts (c) and. To be tautomers, the two species must feature a central carbon which in the enol form. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group.
Alkehydes And Symmetrical Ketones Typically Only Have One Possible Enol Tautomer While Asymmetrical.
This problem has been solved! Web the keto form and the enol form, and these are different molecules.